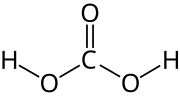
Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water...
21 KB (2,128 words) - 03:22, 30 June 2024
water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site of most carbonic anhydrases contains a zinc ion. They...
38 KB (4,350 words) - 11:09, 19 June 2024
diatomic molecules. Carbon dioxide is soluble in water, in which it reversibly forms H2CO3 (carbonic acid), which is a weak acid, because its ionization...
112 KB (13,042 words) - 17:55, 3 August 2024
buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO− 3), and carbon dioxide (CO2) in...
14 KB (1,723 words) - 07:34, 3 June 2024
+ NaHCO3 → NaCl + H2CO3 The carbonic acid rapidly equilibrates with carbon dioxide and water through catalysis by carbonic anhydrase enzymes bound to the...
15 KB (1,772 words) - 22:35, 9 May 2024
Carbonation (redirect from M-C bond insertion to carbon dioxide)
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in...
4 KB (445 words) - 01:23, 21 January 2024
bicarbonate Calcium bicarbonate Ammonium bicarbonate Carbonic acid Carbon dioxide Carbonate Carbonic anhydrase Hard water Arterial blood gas test "hydrogencarbonate...
13 KB (1,134 words) - 13:27, 31 July 2024
A carbonate is a salt of carbonic acid, H2CO3, characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word "carbonate"...
18 KB (1,689 words) - 17:42, 8 August 2024
considered an oxoacid of carbon. Orthocarbonic acid is highly unstable. Calculations show that it decomposes into carbonic acid and water: H4CO4 → H2CO3...
12 KB (1,035 words) - 13:34, 19 August 2024
derived from metabolic carbon dioxide which is enzymatically converted to carbonic acid in the renal tubular cells. There, carbonic acid spontaneously dissociates...
21 KB (2,420 words) - 13:47, 7 August 2024