chemistry, bond energy (BE) is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy...
9 KB (1,318 words) - 02:44, 29 April 2024
The bond-dissociation energy (BDE, D0, or DH°) is one measure of the strength of a chemical bond A−B. It can be defined as the standard enthalpy change...
23 KB (2,316 words) - 12:13, 9 July 2024
between the two atomic nuclei. Energy is released by bond formation. This is not as a result of reduction in potential energy, because the attraction of the...
40 KB (4,876 words) - 20:13, 24 May 2024
a 1-electron bond is found in the dihydrogen cation, H+ 2. One-electron bonds often have about half the bond energy of a 2-electron bond, and are therefore...
28 KB (3,654 words) - 07:13, 1 April 2024
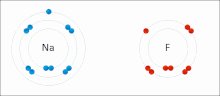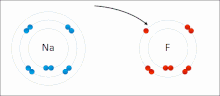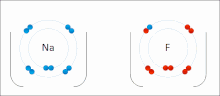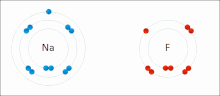
very stable. Ionic bonds have high bond energy. Bond energy is the mean amount of energy required to break the bond in the gaseous state. Most ionic compounds...
18 KB (2,338 words) - 02:36, 8 February 2024
recorded ionization energy for a stable chemical compound. Bond-dissociation energy, the measure of the strength of a chemical bond calculated through...
51 KB (5,883 words) - 15:10, 14 May 2024
intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the...
46 KB (5,417 words) - 02:46, 22 June 2024
that originally contributed to the bond, leading to two unpaired dangling bonds. A dangling bond adds an extra energy level between the valence band and...
25 KB (3,300 words) - 16:45, 20 September 2023
Molecular orbital (redirect from Gamma bond)
which have an energy higher than the energy of their constituent atomic orbitals, and so oppose the bonding of the molecule, and non-bonding orbitals which...
35 KB (4,390 words) - 11:31, 6 June 2024
sigma and one pi bond, has a bond energy less than twice that of a C-C single bond, indicating that the stability added by the pi bond is less than the...
7 KB (816 words) - 06:21, 24 December 2023