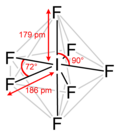
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər,: 410 və-SEP-ər) is a model used in chemistry to predict the geometry...
45 KB (4,038 words) - 12:11, 1 November 2024
levels of s and p orbitals. Bent's rule represents a modification of VSEPR theory for molecules of lower than ideal symmetry. For bonds with the larger...
38 KB (4,250 words) - 13:50, 4 October 2024
Chemical bond (redirect from Bonding theory)
polarity of bonds. The octet rule and VSEPR theory are examples. More sophisticated theories are valence bond theory, which includes orbital hybridization...
40 KB (4,872 words) - 13:33, 22 September 2024
pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to...
23 KB (2,957 words) - 14:53, 17 October 2024
Molecular geometry (section VSEPR table)
angles in the table below are ideal angles from the simple VSEPR theory (pronounced "Vesper Theory")[citation needed], followed by the actual angle for the...
23 KB (2,314 words) - 11:20, 14 September 2024
geometry are sometimes described as sp3 hybridized. The AXE method for VSEPR theory states that the classification is AX3E1. The nitrogen in ammonia has...
4 KB (318 words) - 16:20, 11 February 2024
and methylene (CH2). This geometry is almost always consistent with VSEPR theory, which usually explains non-collinearity of atoms with a presence of...
3 KB (328 words) - 17:23, 26 January 2024
pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the...
8 KB (728 words) - 22:32, 5 April 2024
of steric activity is that of SnCl2, which is bent in accordance with VSEPR theory. Some examples where the lone pair appears to be inactive are bismuth(III)...
9 KB (1,155 words) - 01:57, 27 January 2024
the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory. Bismuth chloride can be synthesized directly by passing chlorine over...
8 KB (592 words) - 18:43, 13 October 2022