Haloalkane (redirect from Halogen derivative)
as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although...
20 KB (2,413 words) - 06:14, 8 September 2024
ClO−4 Oxygen fluoride(s), bromine oxide(s), iodine oxide(s) – analogous oxygen halide and halogen oxides Sulfur fluoride(s), sulfur chloride(s), sulfur bromide(s)...
2 KB (288 words) - 06:04, 28 October 2024
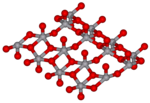
Calcium oxide (formula: CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline...
23 KB (2,231 words) - 17:52, 21 October 2024
Iodine (category Halogens)
element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid...
104 KB (11,657 words) - 18:48, 27 October 2024
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a dark yellow solid, although...
17 KB (1,550 words) - 14:25, 4 October 2024
Oxidizing agent (redirect from Oxidation half reaction)
while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing...
9 KB (875 words) - 16:12, 12 October 2024
acid" refers to hydrated tin (IV) oxide, SnO2, which is also called "stannic oxide." Tin oxides dissolve in acids. Halogen acids attack SnO2 to give hexahalostannates...
15 KB (1,500 words) - 02:45, 19 February 2024
Mark L.; Lineberger, W. C. (1992). "Photoelectron spectroscopy of the halogen oxide anions FO−, ClO−, BrO−, IO−, OClO−, and OIO−". The Journal of Chemical...
2 KB (207 words) - 18:24, 31 August 2024
trace compounds include mercury, halocarbons (including CFCs), and halogen oxide radicals. The abundance of gases varies considerably from volcano to...
23 KB (2,774 words) - 10:20, 5 November 2024