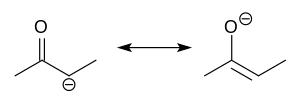
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group R3Si−O−CR=CR2, composed of an enolate...
13 KB (1,369 words) - 19:53, 12 February 2024
used to install silyl groups onto hindered positions. Silyl triflate is more reactive and also converts ketones to silyl enol ethers. Silyl triflates are...
10 KB (1,269 words) - 05:42, 16 January 2024
generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether. Keto–enol tautomerism refers...
14 KB (1,278 words) - 18:45, 24 February 2024
enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are...
7 KB (802 words) - 12:21, 29 May 2024
Ge, Sn, Pb). Such compounds are considered ethers as well. Examples of such ethers are silyl enol ethers R3Si−O−CR=CR2 (containing the Si−O−C linkage)...
19 KB (1,835 words) - 03:40, 19 May 2024
addition is an organic reaction and a type of aldol reaction between a silyl enol ether (R2C=CR−O−Si(CH3)3) and an aldehyde (R−CH=O) or formate (R−O−CH=O)...
9 KB (958 words) - 06:34, 11 February 2024
Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product...
28 KB (3,396 words) - 22:32, 27 March 2024
Self-condensation (section Silyl enol ether formation)
will occur. One way to get around this is to turn the aldehyde into a silyl enol ether using trimethylsilyl chloride and a base, such as triethylamine, and...
4 KB (488 words) - 21:50, 23 October 2023
compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to...
13 KB (1,404 words) - 11:10, 17 January 2023
Silylation (redirect from Silyl)
silylation is to trap silyl enol ethers, which represent a reactive tautomer of many carbonyl compounds. The introduction of a silyl group(s) gives derivatives...
6 KB (586 words) - 04:58, 10 January 2024