Hsp90 (heat shock protein 90) is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in...
51 KB (5,551 words) - 04:57, 26 August 2024
An Hsp90 inhibitor is a substance that inhibits that activity of the Hsp90 heat shock protein. Since Hsp90 stabilizes a variety of proteins required for...
20 KB (2,756 words) - 11:53, 23 April 2024
Chaperone (protein) (section Hsp90)
classified based on their observed molecular weights into Hsp60, Hsp70, Hsp90, Hsp104, and small Hsps. The Hsp60 family of protein chaperones are termed...
29 KB (3,499 words) - 07:16, 20 February 2024
named according to their molecular weight. For example, Hsp60, Hsp70 and Hsp90 (the most widely studied HSPs) refer to families of heat shock proteins...
49 KB (5,533 words) - 10:41, 19 July 2024
suggesting a genetic basis for those phenotypes. The authors hypothesized that Hsp90 (the gene mutated in hsp83), as a chaperone protein, plays a pivotal role...
18 KB (2,035 words) - 18:20, 1 September 2024
connectivity allows Hsp90 to function as a network hub linking diverse protein interaction networks. Within these networks Hsp90 primarily specializes...
65 KB (8,199 words) - 17:04, 1 August 2024
The GHKL domain (Gyrase, Hsp90, Histidine Kinase, MutL) is an evolutionary conserved protein domain. It is an ATPase domain found in several ATP-binding...
7 KB (437 words) - 02:06, 31 July 2023
which acts as co-chaperone of Hsp90 (heat shock protein 90). AHSA2 and the related AHSA1 belongs to the AHA (Activator of Hsp90 ATPase) family of stress-regulated...
1 KB (135 words) - 06:39, 28 March 2022
Stress-induced-phosphoprotein 1 also Hsp70-Hsp90 organising protein (Hop) is encoded in the human by the STIP1 gene. It functions as a co-chaperone which...
16 KB (1,955 words) - 20:23, 3 June 2024
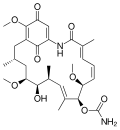
inhibits the function of Hsp90 (Heat Shock Protein 90) by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important...
10 KB (657 words) - 15:24, 2 June 2024