A network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) is a chemical compound (or element) in which...
4 KB (456 words) - 17:52, 16 July 2024
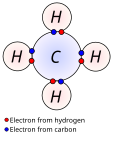
covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal...
28 KB (3,673 words) - 14:01, 24 October 2024
chemistry, a hydrogen bond (or H-bond) is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative...
46 KB (5,459 words) - 23:38, 3 October 2024
which forms network covalent solids (sometimes called simply "covalent solids") Ionic bonding, which forms ionic solids Metallic bonding, which forms...
11 KB (1,382 words) - 14:09, 6 September 2024
complex ion Ag(NH3)2+, which has two Ag←N coordinate covalent bonds. In metallic bonding, bonding electrons are delocalized over a lattice of atoms. By...
40 KB (4,872 words) - 13:33, 22 September 2024
Covalent adaptable networks (CANs) are a type of polymer material that closely resemble thermosetting polymers (thermosets). However, they are distinguished...
25 KB (2,887 words) - 16:07, 23 April 2024
scale) that they form covalent bonds in which the electrons are shared almost equally. Ruthenium boride Network covalent bonding Superhard material Gaidar'...
11 KB (1,052 words) - 18:34, 11 September 2024
Valence electron (category Chemical bonding)
formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing...
24 KB (2,333 words) - 14:12, 27 October 2024
together. Bonding is a mutual, interactive process, and is different from simple liking. It is the process of nurturing social connection. Bonding typically...
32 KB (4,279 words) - 03:16, 16 May 2024