"Prof. Christopher Huber". Biontech Biography. Biontech. Retrieved 5 August 2021. "SEC Filing | BioNTech". investors.biontech.de. p. 130. Retrieved 28 December...
41 KB (3,732 words) - 00:41, 14 October 2024
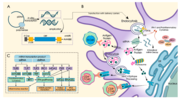
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company...
260 KB (22,097 words) - 23:43, 14 October 2024
Operation Warp Speed (section Pfizer–BioNTech)
at BioNTech's laboratories in Mainz, Germany, just days after the SARS-Cov-2 genetic sequence was first made public. In September 2020, BioNTech received...
71 KB (5,872 words) - 02:08, 19 October 2024
Pfizer–BioNTech's BNT162b2 COVID-19 vaccine for widespread use. On 11 December, the FDA gave emergency use authorization for the Pfizer–BioNTech COVID-19...
77 KB (7,878 words) - 16:22, 10 October 2024
delivery schedule for Pfizer-BioNTech’s COVID-19 vaccine." By the end of September, Canada will receive the 40 million Pfizer-BioNTech doses. Major General Fortin...
185 KB (14,787 words) - 04:28, 26 July 2024
approved the Pfizer-BioNTech vaccine for section 21 Emergency Use Authorisation. A trial ending in March 2021 of the Pfizer-BioNTech's COVID-19 vaccine appeared...
198 KB (13,219 words) - 03:24, 24 October 2024
regulatory authority recognized by the World Health Organization (WHO): Pfizer–BioNTech, Oxford–AstraZeneca, Sinopharm BIBP, Moderna, Janssen, CoronaVac, Covaxin...
322 KB (19,558 words) - 03:33, 16 September 2024
On 25 January 2021, the TGA provisionally approved the two-dose Pfizer–BioNTech COVID-19 vaccine, named COMIRNATY, for use within Australia. The provisional...
24 KB (1,870 words) - 08:58, 23 September 2024
Administration (FDA) first granted emergency use authorization to the Pfizer–BioNTech vaccine on December 10, 2020, and mass vaccinations began four days later...
134 KB (10,719 words) - 20:36, 7 November 2024
Bank to address the health and economic impact of COVID-19. Meanwhile, BioNTech and the European Union are collaborating to assess mRNA vaccine production...
110 KB (11,862 words) - 22:35, 9 November 2023