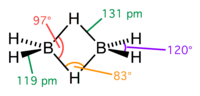
Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively...
27 KB (2,600 words) - 19:52, 29 October 2024
There are other forms of diborane with different numbers of hydrogen atoms, including diborane(4) and diborane(6). Diborane(2) is a highly reactive molecule...
12 KB (1,222 words) - 12:07, 30 September 2024
Diborane(4) is a transient inorganic compound with the chemical formula B 2H 4. Stable derivatives are known. Diborane(4) has been produced by abstraction...
4 KB (299 words) - 04:06, 27 October 2023
chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: BX3 +BH4− → HBX3−...
13 KB (1,243 words) - 14:32, 17 August 2024
structure and bonding. First, new synthetic techniques were required to handle diborane and many of its derivatives, which are both pyrophoric and volatile. Alfred...
5 KB (561 words) - 16:24, 21 October 2024
molecules and ions are described as dimers, even when the monomer is elusive. Diborane (B2H6) is an dimer of borane, which is elusive and rarely observed. Almost...
12 KB (1,298 words) - 11:51, 19 October 2024
electrons in bonding. This type of bonding occurs in boron hydrides such as diborane (B2H6), which are often described as electron deficient because there are...
28 KB (3,673 words) - 14:01, 24 October 2024
hydrogen fuel, but is otherwise primarily of academic interest. Reaction of diborane with ammonia mainly gives the diammoniate salt [H2B(NH3)2]+[BH4]−...
13 KB (1,032 words) - 07:30, 6 September 2024
reactions of diborane, B2H6. Hermann Irving Schlesinger's laboratory at the University of Chicago was one of two laboratories that prepared diborane. It was...
15 KB (1,386 words) - 05:55, 9 June 2024
and will burn readily (e.g., gasoline, acetylene, propane, hydrogen gas, diborane). Includes pyrophoric substances. Flash point below room temperature at...
14 KB (680 words) - 15:41, 17 October 2024