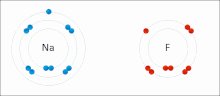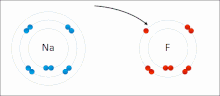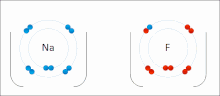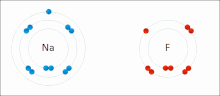
In chemistry, the lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed...
10 KB (1,205 words) - 20:26, 20 April 2024
of calculating the lattice energy of a crystalline ionic compound. In 1918 Max Born and Alfred Landé proposed that the lattice energy could be derived from...
5 KB (838 words) - 21:46, 19 July 2024
effect on their bond energy. The extent of this effect is described by the compound's lattice energy, where a more negative lattice energy corresponds to a...
9 KB (1,318 words) - 02:44, 29 April 2024
other releases (lattice) energy and, thus, lowers the overall energy of the system. Ionic bonding will occur only if the overall energy change for the...
18 KB (2,338 words) - 02:36, 8 February 2024
crystalline solid comprises the hydration energy. If the hydration energy is greater than the lattice energy, then the enthalpy of solution is negative...
3 KB (345 words) - 16:35, 28 September 2023
Brillouin zone (redirect from Reciprocal lattice primitive cell)
In the same way the Bravais lattice is divided up into Wigner–Seitz cells in the real lattice, the reciprocal lattice is broken up into Brillouin zones...
11 KB (639 words) - 12:17, 16 September 2023
amount of heat (known as the lattice energy) to the surroundings. Although heat is "wasted" energy for a specific energy transfer (see: waste heat), it...
59 KB (7,466 words) - 21:47, 13 August 2024
Band gap (redirect from Band gap energy)
the lattice phonons and the free electrons and holes will also affect the band gap to a smaller extent. The relationship between band gap energy and temperature...
23 KB (2,484 words) - 00:28, 11 June 2024
a reasonable form is assumed for the additional repulsive energy, the total lattice energy can be modelled using the Born–Landé equation, the Born–Mayer...
63 KB (6,942 words) - 13:02, 22 August 2024
a means of calculating lattice energy (or more precisely enthalpy), which cannot otherwise be measured directly. The lattice enthalpy is the enthalpy...
7 KB (941 words) - 22:25, 17 December 2023