Oswald Silberrad. Its solid state structure consists of chains of -NI2-I-NI2-I-NI2-I-. Ammonia molecules are situated between the chains. When kept cold...
8 KB (698 words) - 11:59, 8 June 2024
in which Li+ substitutes Ni2+ ions in the lattice, increases as nickel concentration increases as well. The similar size of Ni2+ (0.69 Å) and Li+ (0.76...
22 KB (2,377 words) - 12:31, 8 August 2024
general base used in the Blakely mechanism, His320, was too far away from the Ni2-bound water to deprotonate in order to form the attacking hydroxide moiety...
34 KB (4,155 words) - 22:51, 3 July 2024
NiCoT family (redirect from Ni2+-Co2+ Transporter)
Proteins currently known to belong to the Ni2+-Co2+ Transporter (NiCoT) family (TC# 2.A.52) can be found in organisms ranging from Gram-negative and Gram-positive...
5 KB (550 words) - 07:35, 16 April 2022
Gwoyeu Romatzyh Tsao Ni Maa Wade–Giles Ts'ao3 ni2 ma3 Tongyong Pinyin Cǎo ní mǎ Yale Romanization Tsau3 ni2 ma3 MPS2 Tsǎu-ní-mǎ Hakka Romanization Chhó...
23 KB (2,479 words) - 00:20, 4 October 2024
Manganin (section Cu86/Mn12/Ni2)
Manganin is a trademarked name for an alloy of typically 84.2% copper, 12.1% manganese, and 3.7% nickel. It was first developed by Edward Weston in 1892...
8 KB (451 words) - 20:20, 6 October 2024
ammonia/ammonium chloride solution used to detect group 3 cations. It includes: Zn2+, Ni2+, Co2+, and Mn2+. Zinc will form a white precipitate, nickel and cobalt a...
24 KB (2,719 words) - 11:38, 26 September 2024
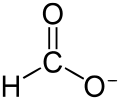
hydrated nickel formate decarboxylates at about 200 °C with reduction of the Ni2+ to finely powdered nickel metal: Ni(HCO2)2(H2O)2 → Ni + 2 CO2 + 2 H2O +...
6 KB (528 words) - 14:19, 3 September 2024
NiSO4(H2O)6. This highly soluble turquoise coloured salt is a common source of the Ni2+ ion for electroplating. Approximately 40,000 tonnes were produced in 2005...
11 KB (1,004 words) - 13:46, 6 October 2024