Lithium peroxide is the inorganic compound with the formula Li2O2. Lithium peroxide is a white solid, and unlike most other alkali metal peroxides, it...
7 KB (527 words) - 14:36, 21 July 2024
decompose lithium peroxide into lithium ions, thereby charging the battery. During discharge, oxygen from air replenished the lithium peroxide. List of...
51 KB (5,671 words) - 23:20, 6 November 2024
the principal lithium mineral spodumene (LiAlSi2O6) is 8.03%. Lithium oxide forms along with small amounts of lithium peroxide when lithium metal is burned...
7 KB (480 words) - 18:59, 8 March 2024
useful in scuba gear, submarines, etc. Lithium peroxide and potassium superoxide have similar uses. Sodium peroxide was once used on a large scale for the...
8 KB (590 words) - 13:15, 13 October 2024
hygroscopic and are used as desiccants for gas streams. Lithium hydroxide and lithium peroxide are the salts most commonly used in confined areas, such...
141 KB (13,748 words) - 10:30, 2 November 2024
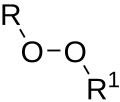
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (R−O−O−R′). If the R′ is hydrogen, the compounds...
23 KB (2,457 words) - 12:03, 30 May 2024
Press. ISBN 0-8493-0487-3. Khosravi J (2007). Production of Lithium Peroxide and Lithium Oxide in an Alcohol Medium. Chapter 9: Results. ISBN 978-0-494-38597-5...
14 KB (1,096 words) - 12:21, 25 August 2024
superoxide. It is currently unclear whether lithium should react analogously. Lithium oxide Lithium peroxide Andrews, Lester (1969-05-15). "Infrared Spectrum...
11 KB (1,208 words) - 20:58, 8 December 2023
Rubidium peroxide is rubidium's peroxide with the chemical formula Rb2O2. Rubidium peroxide can be produced by rapidly oxidizing rubidium in liquid ammonia...
2 KB (190 words) - 16:59, 10 January 2024
Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2. It can be formed from caesium metal...
4 KB (344 words) - 22:31, 12 April 2024