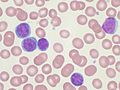
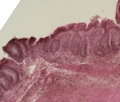
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that inhibits B-cell proliferation and survival by irreversibly...
29 KB (2,605 words) - 21:22, 16 August 2024
adverse effects relative to ibrutinib. In pre-clinical studies, it was shown to be more potent and selective than ibrutinib, the first-in-class BTK inhibitor...
14 KB (1,047 words) - 03:31, 31 August 2024
underlying disease. Ibrutinib is another agent that has been approved for use in this condition. Combination treatment with ibrutinib and rituximab showed...
59 KB (5,773 words) - 01:40, 24 October 2024
well as potential for combination is expected from other drugs such as ibrutinib and idelalisib, both of which were also approved in 2014 to treat CLL...
27 KB (2,575 words) - 15:22, 28 October 2024
brigatinib, baricitinib, cabozantinib, dasatinib, neratinib, eltrombopag, ibrutinib, lenvatinib, palbociclib, regorafenib, tofacitinib, and trelagliptin....
5 KB (232 words) - 10:23, 8 March 2024
first line treatments may be offered. As of 2021, BTK inhibitors such as ibrutinib and acalabrutinib are often recommended for first line treatment of CLL...
73 KB (7,597 words) - 22:02, 28 August 2024
inhibits BTK in a way that is different from the prototypical BTK inhibitor ibrutinib by binding in a different way that avoids a genetic change (mutation at...
17 KB (1,165 words) - 07:22, 22 August 2024
pentostatin, etanercept, and alemtuzumab. In August 2017, the US FDA approved ibrutinib to treat chronic GvHD after failure of one or more other systemic treatments...
39 KB (4,442 words) - 15:41, 17 October 2024
September 2021. Retrieved 2021-10-01. "Summary of opinion: Imbruvica,ibrutinib". Case Medical Research. 2019-06-28. doi:10.31525/cmr-1658a62. ISSN 2643-4652...
25 KB (1,860 words) - 04:49, 26 July 2024
acquire oncology firm Pharmacyclics and its treatment for blood cancers, ibrutinib; AstraZeneca had also been bidding to acquire Pharmacyclics. Under the...
42 KB (3,542 words) - 10:02, 1 November 2024