α-Amanitin
 | |
 | |
| Names | |
|---|---|
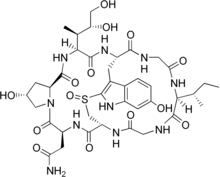
| Other names (cyclic L-asparaginyl-4-hydroxy-L-proly-(R)-4,5-dihydroxy-L-isoleucyl-6-hydroxy-2-mercapto-L-tryptophylglycyl-L-isoleucylglycyl-L-cysteinyl) cyclic (4 → 8)-sulfide(R)-S-oxide. | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.287 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C39H54N10O14S | |
| Molar mass | 918.97 g/mol |
| Good | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Highly toxic |
| GHS labelling: | |
 | |
| H300, H310, H330, H373 | |
| P260, P262, P264, P270, P271, P280, P284, P301+P310, P302+P350, P304+P340, P310, P314, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
α-Amanitin (alpha-Amanitin) is a cyclic peptide of eight amino acids. It is possibly the most deadly of all the amatoxins, toxins found in several species of the mushroom genus Amanita, one being the death cap (Amanita phalloides) as well as the destroying angel, a complex of similar species, principally A. virosa and A. bisporigera. It is also found in the mushrooms Galerina marginata, Lepiota subincarnata and Conocybe filaris. The oral LD50 of amanitin is 100 μg/kg for rats.
Unlike most cyclic peptides, amatoxins (and phallotoxins) are synthesized on ribosomes. The genes encoding the proprotein for α-amanitin belong to the same family as those that encode for phallacidin (a phallotoxin).[1]
Scientific use
[edit]α-Amanitin is a selective inhibitor of RNA polymerase II and III but not I.[2][3] This mechanism makes it a deadly toxin.
α-Amanitin can also be used to determine which types of RNA polymerase are present. This is done by testing the sensitivity of the polymerase in the presence of α-amanitin. RNA polymerase I is insensitive, RNA polymerase II is highly sensitive (inhibited at 1μg/ml), RNA polymerase III is moderately sensitive (inhibited at 10μg/ml), and RNA polymerase IV is slightly sensitive (inhibited at 50μg/ml).[citation needed][4][5]
Chemical structure
[edit]α-amanitin is a highly modified bicyclic octapeptide consisting of an outer and an inner loop. The outer loop is formed by peptide bonds between a carboxyl terminus of an amino acid to the subsequent amino terminus of the next residue. The inner loop is closed by a tryptathionine linkage between 6-hydroxy-tryptophan and cysteine. In addition, α-amanitin is decorated with modified amino acid side chains (2S,3R,4R)-4,5-dihydroxy-isoleucine, trans-4-hydroxy-proline, which gives its high affinity for RNA polymerase II and III.[6]
Detection techniques
[edit]Early methods to detect alpha-amanitin included thin-layer chromatography (TLC). In most solvent systems used in TLC, alpha-amanitin and beta-amanitin would travel at different rates, thus allowing individual identification of each toxin. Another early method was the Meixner test (also known as the Wieland test), which would detect amatoxins, but also yielded false positives for some compounds, such as psilocin.[7] Capillary zone electrophoresis was also developed, but was not adequately sensitive for clinical samples, but sufficient for mushroom extracts.[8]
More recently, the use of high-performance liquid chromatography (HPLC) has become the preferred method, which allows for better resolution, reproducibility, and higher sensitivity.[9] A range of detectors can be paired with HPLC, such as UV or mass spectrometry.
As early as the 1980s, antibody-based assays (immunoassays) were developed for amanitin (but more often recognize amatoxins as the antibodies cross-react with some of the congeners). The earliest immunoassays were radioimmunoassays and then enzyme linked immunosorbent assays (ELISAs). More, recently, in 2020, a monoclonal antibody-based lateral flow immunoassay (similar to a pregnancy test) has been developed that can quickly and selectively detect amatoxins in mushrooms[10] and in urine samples.[11]
Total synthesis
[edit]Matinkhoo et al. devised strategies to surmount three synthetic hurdles to give α-amanitin in 2018.[12] First, enantioselective synthesis of solid phase peptide synthesis-compatible (2S,3R,4R)-4,5-dihydroxyisoleucine was afforded in 11 steps from 2-(benzyloxy)acetaldehyde. Two key stereochemistry-defining steps include Brown crotylation at (3R,4R)-positions, and asymmetric Strecker amino acid synthesis at the (2S)-α carbon.[13] Secondly, chemoselective inner ring closure by fluorocyclization between 6-hydroxytrytophan and cysteine was achieved by intra-annular Savige-Fontana reaction. This requires a solid phase peptide synthesis-compatible, and methyliminodiacetic acid (MIDA), a boron protecting group, orthogonal amino acid in 5 steps.[12] As a final step, enantioselective oxidation at the tryptathionine linkage was achieved using a bulky organic oxidizing agent and an optimized solvent system to afford the desired bio-reactive (R)-enantiomer sulfoxide, completing the total synthesis.[citation needed]
Symptoms of poisoning
[edit]α-Amanitin has an unusually strong and specific attraction to the enzyme RNA polymerase II. Upon ingestion and uptake by liver cells, it binds to the RNA polymerase II enzyme, effectively causing cytolysis of hepatocytes (liver cells).[14] Few effects are reported within 10 hours; it is not unusual for significant effects to take as long as 24 hours after ingestion to appear, with this delay in symptoms making α-amanitin poisoning even more difficult to diagnose and all the more dangerous. By then, it is far past the time in which stomach pumping would yield an efficient result. Diarrhea and cramps are the first symptoms, but those pass, giving a false sign of remission. Typically, on the 4th to 5th day, the toxin starts to have severe effects on the liver and kidneys, leading to total system failure in both. Death usually takes place around a week from ingestion.[15]
Around 15% of those poisoned will die within 10 days, progressing through a comatose stage to kidney failure, liver failure, hepatic coma, respiratory failure and death. Those who recover are at risk of permanent liver damage.[16] Diagnosis is difficult, and is established by observation of the clinical symptoms as well as the presence of α-amanitin in the urine. Urine screening is generally most useful within 48 hours of ingestion. Treatment is mainly supportive (gastric lavage, activated carbon, fluid resuscitation) but includes various drugs to counter the amatoxins, including intravenous penicillin and cephalosporin derivatives, and, in cases of greater ingestion, can extend to an orthotopic liver transplant. The most reliable method to treat amanitin poisoning is through gastric lavage immediately after ingestion; however, the onset of symptoms is generally too late for this to be an option. Chemically modified silibinin, silibinin dihydrogen disuccinate disodium (trade name Legalon SIL) a solution for IV administration, is used in treatment of severe intoxications with hepatotoxic substances such as paracetamol and amanitins.[17]
Mode of inhibitory action
[edit]
Based on a 2002 crystal structure analysis, α-amanitin interacts with the bridge helix in RNA polymerase II (pol II).[18] This interaction interferes with the translocation of RNA and DNA needed to empty the site for the next round of RNA synthesis. The addition of α-amanitin can reduce the rate of pol II translocating on DNA from several thousand to a few nucleotides per minute,[19][20] but has little effect on the affinity of pol II for nucleoside triphosphate,[21] and a phosphodiester bond can still be formed.[22][23] The bridge helix has evolved to be flexible and its movement is required for translocation of the polymerase along the DNA backbone. Binding of α-amanitin puts a constraint on its mobility, hence slowing down the translocation of the polymerase and the rate of synthesis of the RNA molecule.[citation needed]
Use in antibody-drug conjugates
[edit]A new antibody-drug conjugate (ADC) technology based on α-amanitin has shown activity in therapy-resistant tumor cells, e.g. cells expressing multi-drug resistant transporters, tumor-initiating cells and non-dividing cells at picomolar concentrations.[24]
The unique mode of action of α-amanitin seems to make the amanitin-based ADCs a suitable toxic payload.[25] Amanitin has a water-soluble structure, resulting in ADCs with low tendency for aggregation.[26][27]
See also
[edit]References
[edit]- ^ Hallen HE, Luo H, Scott-Craig JS, Walton JD (November 2007). "Gene family encoding the major toxins of lethal Amanita mushrooms". Proceedings of the National Academy of Sciences of the United States of America. 104 (48): 19097–101. Bibcode:2007PNAS..10419097H. doi:10.1073/pnas.0707340104. PMC 2141914. PMID 18025465.
- ^ ADC Review Team (2019-03-23). "What is Alpha-Amanitin?". Editorial. ADC Review. Retrieved 2020-04-17.
- ^ Meinecke B, Meinecke-Tillmann S (May 1993). "Effects of alpha-amanitin on nuclear maturation of porcine oocytes in vitro". Journal of Reproduction and Fertility. 98 (1): 195–201. doi:10.1530/jrf.0.0980195. PMID 8345464.
- ^ Gao Z, Herrera-Carrillo E, Berkhout B (September 2018). "RNA Polymerase II Activity of Type 3 Pol III Promoters". Molecular Therapy: Nucleic Acids. 12: 135–145. doi:10.1016/j.omtn.2018.05.001. PMC 6023835. PMID 30195753.
- ^ Latchman D (2018-03-29). Gene Control. Garland Science. ISBN 9781136844201.
- ^ Meinecke B, Meinecke-Tillmann S (May 1993). "Effects of alpha-amanitin on nuclear maturation of porcine oocytes in vitro". Journal of Reproduction and Fertility. 98 (1): 195–201. doi:10.1530/jrf.0.0980195. PMID 8345464.
- ^ Beuhler M, Lee DC, Gerkin R (August 2004). "The Meixner test in the detection of alpha-amanitin and false-positive reactions caused by psilocin and 5-substituted tryptamines". Annals of Emergency Medicine. 44 (2): 114–20. doi:10.1016/j.annemergmed.2004.03.017. PMID 15278082.
- ^ Brüggemann O, Meder M, Freitag R (September 1996). "Analysis of amatoxins alpha-amanitin and beta-amanitin in toadstool extracts and body fluids by capillary zone electrophoresis with photodiode array detection". Journal of Chromatography A. 8th International Symposium on High Performance Capillary Electrophoresis Part I. 744 (1–2): 167–76. doi:10.1016/0021-9673(96)00173-2. PMID 8843665.
- ^ Walton J (9 May 2018). The cyclic peptide toxins of Amanita and other poisonous mushrooms. Cham, Switzerland. ISBN 978-3-319-76822-9. OCLC 1035556400.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Bever CS, Adams CA, Hnasko RM, Cheng LW, Stanker LH (2020-04-17). "Lateral flow immunoassay (LFIA) for the detection of lethal amatoxins from mushrooms". PLOS ONE. 15 (4): e0231781. Bibcode:2020PLoSO..1531781B. doi:10.1371/journal.pone.0231781. PMC 7164595. PMID 32302363.
- ^ Bever CS, Swanson KD, Hamelin EI, Filigenzi M, Poppenga RH, Kaae J, et al. (February 2020). "Rapid, Sensitive, and Accurate Point-of-Care Detection of Lethal Amatoxins in Urine". Toxins. 12 (2): 123. doi:10.3390/toxins12020123. PMC 7076753. PMID 32075251.
- ^ a b Matinkhoo K, Pryyma A, Todorovic M, Patrick BO, Perrin DM (May 2018). "Synthesis of the Death-Cap Mushroom Toxin α-Amanitin". Journal of the American Chemical Society. 140 (21): 6513–6517. doi:10.1021/jacs.7b12698. PMID 29561592.
- ^ Mohapatra DK, Das PP, Pattanayak MR, Yadav JS (February 2010). "Iodine-catalyzed highly diastereoselective synthesis of trans-2,6-disubstituted-3,4-dihydropyrans: application to concise construction of C28-C37 bicyclic core of (+)-sorangicin A". Chemistry: A European Journal. 16 (7): 2072–8. doi:10.1002/chem.200902999. PMID 20099288.
- ^ Michelot D, Labia R (1988). "alpha-Amanitin: a possible suicide substrate-like toxin involving the sulphoxide moiety of the bridged cyclopeptide". Drug Metabolism and Drug Interactions. 6 (3–4): 265–74. doi:10.1515/dmdi.1988.6.3-4.265. PMID 3078291. S2CID 23872903.
- ^ Mas A (February 2005). "Mushrooms, amatoxins and the liver". Journal of Hepatology. 42 (2): 166–9. doi:10.1016/j.jhep.2004.12.003. PMID 15664239.
- ^ Benjamin DR (1995). "Amatoxin syndrome". Mushrooms: poisons and panaceas — a handbook for naturalists, mycologists and physicians. New York: WH Freeman and Company. pp. 198–214.
- ^ Clinical trial number NCT00915681 for "Intravenous Milk Thistle (Silibinin-Legalon) for Hepatic Failure Induced by Amatoxin/Amanita Mushroom Poisoning" at ClinicalTrials.gov
- ^ a b Bushnell DA, Cramer P, Kornberg RD (February 2002). "Structural basis of transcription: alpha-amanitin-RNA polymerase II cocrystal at 2.8 A resolution". Proceedings of the National Academy of Sciences of the United States of America. 99 (3): 1218–22. Bibcode:2002PNAS...99.1218B. doi:10.1073/pnas.251664698. PMC 122170. PMID 11805306.
- ^ Chafin DR, Guo H, Price DH (August 1995). "Action of alpha-amanitin during pyrophosphorolysis and elongation by RNA polymerase II". The Journal of Biological Chemistry. 270 (32): 19114–9. doi:10.1074/jbc.270.32.19114. PMID 7642577.
- ^ Rudd MD, Luse DS (August 1996). "Amanitin greatly reduces the rate of transcription by RNA polymerase II ternary complexes but fails to inhibit some transcript cleavage modes". The Journal of Biological Chemistry. 271 (35): 21549–58. doi:10.1074/jbc.271.35.21549. PMID 8702941.
- ^ Cochet-Meilhac M, Chambon P (June 1974). "Animal DNA-dependent RNA polymerases. 11. Mechanism of the inhibition of RNA polymerases B by amatoxins". Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis. 353 (2): 160–84. doi:10.1016/0005-2787(74)90182-8. PMID 4601749.
- ^ Vaisius AC, Wieland T (June 1982). "Formation of a single phosphodiester bond by RNA polymerase B from calf thymus is not inhibited by alpha-amanitin". Biochemistry. 21 (13): 3097–101. doi:10.1021/bi00256a010. PMID 7104312.
- ^ Gu W, Powell W, Mote J, Reines D (December 1993). "Nascent RNA cleavage by arrested RNA polymerase II does not require upstream translocation of the elongation complex on DNA". The Journal of Biological Chemistry. 268 (34): 25604–16. doi:10.1016/S0021-9258(19)74433-0. PMC 3373964. PMID 7503982.
- ^ "Alpha Amanitin". ADC Review / Journal of Antibody-drug Conjugates. ISSN 2327-0152. Retrieved 26 May 2017.
- ^ ADC Review Editorial Team. "What are antibody-drug conjugates?". ADC Review / Journal of Antibody-drug Conjugates. ISSN 2327-0152. Retrieved 26 May 2017.
- ^ Moldenhauer G, Salnikov AV, Lüttgau S, Herr I, Anderl J, Faulstich H (April 2012). "Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma". Journal of the National Cancer Institute. 104 (8): 622–34. doi:10.1093/jnci/djs140. PMID 22457476.
- ^ Hechler T, Kulke M, Müller C, Pahl A, Anderl J (2014). Amanitin-based antibody-drug conjugates targeting the prostate-specific membrane antigen PSMA. Poster #664. AACR Annual Meeting. doi:10.1158/1538-7445.AM2014-664.


 French
French Deutsch
Deutsch