Adipiplon
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
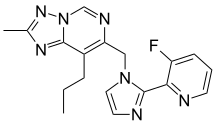
| Formula | C18H18FN7 |
| Molar mass | 351.389 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Adipiplon (developmental code name NG2-73) is an anxiolytic drug developed by Neurogen Corporation. It has similar effects to benzodiazepine drugs, but is structurally distinct and classed as a nonbenzodiazepine anxiolytic.
Adipiplon is a subtype-selective GABAA receptor partial agonist, which binds preferentially to the α3 subtype. This is significant as while several previous nonbenzodiazepine drugs have been developed that are selective for α2/3 over the other subtypes, adipiplon is one of the first drugs selected for clinical development which can discriminate between α2 and α3, as well as showing a little affinity for the α1 or α5 subtypes — alpidem is selective for α3 over α2, but still has moderate affinity for α1, whereas adipiplon is highly α3-selective with little affinity for either α1, α2 or α5.
Adipiplon was being researched as a potential medication for the treatment of anxiety and insomnia, and in 2008 it was being used in Phase IIb trials.[1][2][3] These trials were suspended after significant next-day side effects were discovered.[4]
See also
[edit]References
[edit]


 French
French Deutsch
Deutsch