Bailey peptide synthesis
The Bailey peptide synthesis is a name reaction in organic chemistry developed 1949 by J. L. Bailey.[1][2] It is a method for the synthesis of a peptide from α-amino acid-N-carboxylic acid anhydrides (NCAs) and amino acids or peptide esters.[2][3] The reaction is characterized by short reaction times and a high yield of the target peptide.[2]
The reaction can be carried out at low temperatures in organic solvents.[2] The residues R1 and R2 can be organic groups or hydrogen atoms, R3 is the used amino acid or peptide ester:[2]

Reaction mechanism
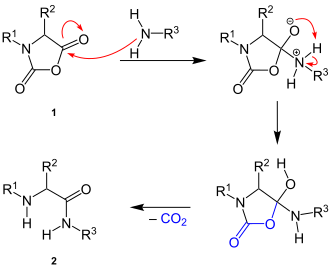
[edit]The reaction mechanism is not known in detail. Supposedly, the reaction begins with a nucleophilic attack of the amino group on the carbonyl carbon of the anhydride group of the N-carboxylic acid anhydride (1). After an intramolecular proton migration, a 1,4-proton shift and the cleavage of carbon dioxide follows, resulting in the peptide bond in the final product (2):[2]
Atom economy
[edit]The advantage in atom economy of using NCAs for peptide formation is that there is no need for a protecting group on the functional group reacted with the amino acid.[4] For example, the Merrifield synthesis depends on the use of Boc and Bzl protecting groups, which need be removed after the reaction.[5] In the case of Bailey peptide synthesis, the free peptide is directly obtained after the reaction.[4] However, unwanted and difficult to remove by-products may be formed.[4] An N-substitution of the NCA (for example, by an o-nitrophenylsulfenyl group) can simplify the subsequent purification process, but on the other hand deteriorates the atom economy of the reaction. [4] The synthesis of NCAs can be carried out by the Leuchs reaction[6] or by the reaction of N-(benzyloxycarbonyl)-amino acids with oxalyl chloride.[7] In the latter case, again the procedure is less efficient in the sense of atom economy.
Synthesized peptides
[edit]The following peptides were synthesized using this method by 1949:[3]
- DL-Ala-Gly
- L-Tyr-Gly
- DL-Tyr-Tyr
- DL-Ala-DL-Ala-Gly
- DL-di-Ala-L-cystinyl-di-Gly
- DL-Ala-L-Tyr-Gly
- DL-Ala-L-Tyr-Gly-Gly
- DL-Ala-DL-Ala-L-Tyr-Gly-Gly
- L-Tyr-L-Tyr-L-Tyr
- L-Cystinyl-di-Gly
Literature
[edit]- P. Katsoyannis: The Chemistry of Polypeptides. Springer Science & Business Media, 2012, ISBN 978-1-461-34571-8, S. 129.
References
[edit]- ^ J. L. Bailey: A new peptide synthesis. In: Nature. Band 164, Nummer 4177, November 1949, S. 889, PMID 15407090.
- ^ a b c d e f Daniel Zerong Wang (2009), Comprehensive Organic Name Reactions and Reagents, vol. 1, Hoboken New Jersey: John Wiley & Sons, Inc., pp. 156–159, ISBN 978-0-471-70450-8
- ^ a b J. Leggett Bailey: A new Peptide Synthesis. In: Nature. Band 164, Nr. 4177, 1949, S. 889, DOI:10.1038/164889a0.
- ^ a b c d Ryoichi Katakai: Peptide Synthesis Using o-Nitrophenylsulfenyl N-Carboxy α-Amino Acid Anhydrides. In: The Journal of Organic Chemistry., Band 40, Nr. 19, 1975, S. 2697–2702, DOI:10.1021/jo00907a001.
- ^ Hans-Dieter Jakubke (1996), Peptide. Chemie und Biologie (in German), Heidelberg Berlin Oxford: Spektrum Akademischer Verlag GmbH, p. 178, ISBN 3-8274-0000-7
- ^ Hans Rytger Kricheldorf: α-Aminoacid-N-Carboxy-Anhydrides and Related Heterocycles. Syntheses, Properties, Peptide Synthesis, Polymerization. Springer Verlag, Berlin Heidelberg, 1987, S. 1–4, DOI: 10.1007/978-3-642-71586-0, ISBN 978-3-642-71588-4 (Druck), ISBN 978-3-642-71586-0 (Online).
- ^ D. Konopinska, I.Z. Siemion: Synthesis of N-Carboxy-α-amino Acid Anhydrides. In: Angewandte Chemie International Edition. Band 6, Nr. 3, 1967, S. 248, DOI:10.1002/anie.196702481.


 French
French Deutsch
Deutsch