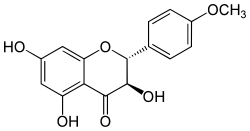
Dihydrokaempferide
 | |
| Names | |
|---|---|
| IUPAC name 3,5,7-trihydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C16H14O6 | |
| Molar mass | 302.27 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dihydrokaempferide is a flavanonol, a type of flavonoid. It can be found in Prunus domestica[1] (plum tree), in the wood of Salix caprea[2] (goat willow) and in the Brazilian green propolis.[3]
References
[edit]- ^ Parmar, Virinder S.; Vardhan, Anand; Nagarajan, G.R.; Jain, Rajni (1992). "Dihydroflavonols from Prunus domestica". Phytochemistry. 31 (6): 2185–6. doi:10.1016/0031-9422(92)80399-Y. INIST 4305555.
- ^ Malterud, Karl E.; Bremnes, Torgunn E.; Faegri, Agnete; Moe, Turid; Dugstad, Eva K. Sandanger; Anthonsen, Thorleif; Henriksen, Liv M. (1985). "Flavonoids from the Wood of Salix caprea as Inhibitors of Wood-Destroying Fungi". Journal of Natural Products. 48 (4): 559–63. doi:10.1021/np50040a007. INIST 8569626.
- ^ Maruyama, Hiroe; Sumitou, Yoshiki; Sakamoto, Takashi; Araki, Yoko; Hara, Hideaki (2009). "Antihypertensive Effects of Flavonoids Isolated from Brazilian Green Propolis in Spontaneously Hypertensive Rats". Biological & Pharmaceutical Bulletin. 32 (7): 1244–50. doi:10.1248/bpb.32.1244. PMID 19571393. INIST 21802341.


 French
French Deutsch
Deutsch