Diiron propanedithiolate hexacarbonyl
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
PubChem CID | |
| |
| |
| Properties | |
| C9H6Fe2O6S2 | |
| Molar mass | 385.83 |
| Appearance | red solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
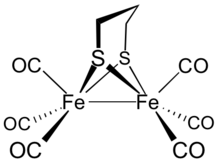
Diiron propanedithiolate hexacarbonyl is the organoiron complex with the formula Fe2(S2C3H6)(CO)6. It is a red diamagnetic solid.[1] It adopts a symmetrical structure with six terminal CO ligands.[2] The complex is a precursor to hydrogenase mimics.
It is prepared by the reaction of 1,3-propanedithiol with triiron dodecarbonyl:
- 2 Fe3(CO)12 + 3 C3H6(SH)2 → 3 Fe2(S2C3H6)(CO)6 + 3 H2 + 6 CO
In general, the CO ligands can be substituted by cyanide, phosphines, isocyanides, N-heterocyclic carbenes, and other donor ligands. Monosubstitution can be achieved through an in situ generation of the acetonitrile complex.[3][4]
Upon irradiation of Fe2(S2C3H6)(CO)6 with ultraviolet (UV) light, CO-photolysis occurs with the transient formation of the unsaturated species followed by the formation of the solvent adduct.[5]
References
[edit]- ^ Winter, A., Zsolnai, L., Huttner, G., "Zweikernige und dreikernige Carbonyleisenkomplexe mit 1,2- und 1,3-Dithiolatobrückenliganden", Z. Naturforsch. 1982, 37b, 1430.
- ^ Lyon, E. J., Georgakaki, I. P., Reibenspies, J. H., Darensbourg, M. Y., "Carbon Monoxide and Cyanide Ligands in a Classical Organometallic Complex Model for Fe-Only Hydrogenase", Angew. Chem. Int. Ed. 1999, 38, 3178.
- ^ Mack, A.; Rauchfuss, T. B., "(1,3-Propanedithiolato)hexacarbonyl and Cyanide Derivatives", Inorganic Synthesis. 2010, volume 35, 143. doi:10.1002/9780470651568.ch7
- ^ Works, C., "Laboratory Experiment or Mini-Project for Inorganic Chemistry or Integrated Laboratory", Journal of Chemical Education. 2007, 84, 836.
- ^ Mack, A. R. Ridley, A. I. Stewart, K. Adamczyk, H. N. Ghosh, B. Kerkeni, Z. X. Guo, E. T. J. Nibbering, C. J. Pickett, N. T. Hunt "(1,3-Propanedithiolato)hexacarbonyl and Cyanide Derivatives", Inorg. Chem. 2008, 47, 7453-7455 doi:10.1021/ic800568k


 French
French Deutsch
Deutsch