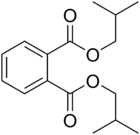
Diisobutyl phthalate
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Bis(2-methylpropyl) benzene-1,2-dicarboxylate | |
| Other names Diisobutyl phthalate Di-iso-butyl phthalate Di(i-butyl)phthalate Diisobutyl ester of phthalic acid 1,2-benzenedicarboxylic acid Bis(2-methylpropyl)ester Di(isobutyl) 1,2-benzenedicarboxylate Isobutyl-O-phthalate DIBP DiBP Palatinol IC | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.412 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C16H22O4 | |
| Molar mass | 278.348 g·mol−1 |
| Appearance | Colorless viscous liquid |
| Density | 1.038 g/cm3 |
| Melting point | −37 °C (−35 °F; 236 K) |
| Boiling point | 320 °C (608 °F; 593 K) |
| 1 mg/L at 20 °C | |
| log P | 4.11 |
| Vapor pressure | 0.01 Pa at 20 °C |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H360Df | |
| P201, P202, P281, P308+P313, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 185 °C (365 °F; 458 K) c.c. |
| 400 °C (752 °F; 673 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Diisobutyl phthalate (DIBP) is a phthalate ester having the structural formula C6H4(COOCH2CH(CH3)2)2. It is formed by the esterification of isobutanol and phthalic anhydride. This and other phthalates are used as plasticizers due to their flexibility and durability. They are found in many industrial and personal products, such as lacquers, nail polish and cosmetics.[1] DIBP can be absorbed via oral ingestion and dermal exposure.[2] When it comes to excretion, DIBP is first converted into the hydrolytic monoester monoisobutyl phthalate (MIBP). The primary excretory route is urine, with biliary excretion being noted in minor amounts. DIBP has lower density and freezing point than the related compound dibutyl phthalate (DBP).[2]
Industry use
[edit]It is used as a plasticizer additive in a range of plastic and rubber materials.[2] It has low volatility, which makes it ideal for use in products that require long-lasting flexibility, e.g. automotive parts, wire and cable insulation, and flooring.[3] It is dense and water-insoluble.[4]
DIBP has been found to be relatively non-toxic, but high levels of exposure to the compound may cause irritation to the eyes, skin and respiratory tract.[2] However, in recent years, concerns have been raised about the potential health risks of exposure to phthalates, including DIBP. Therefore, several countries have restricted or even banned the use of certain phthalates in products.[3] DIBP has been detected in various environmental matrices, such as air, water, and sediment. DIBP is known to bioaccumulate in certain aquatic species[5]
Synthesis
[edit]DIBP is synthesized by reaction of phthalic anhydride with isobutanol. Various acids are used as a catalyst, such as sulfuric acid, sulfonated graphene, or iron(III) chloride. Water is a byproduct.
Using sulfuric acid, the yield is 61% yield.[6]

Optimization
[edit]Sulfonated graphene is a heterogeneous catalyst that has several advantages over traditional liquid acids like sulfuric acid.[7] Sulfonated graphene can be easily separated from the reaction mixture by filtration and can be reused multiple times without reduction in activity.[8] Furthermore, sulfonated graphene is environmentally friendly, as it does not produce hazardous waste materials that are typically generated during the use of traditional liquid acid catalysts. This method has a 95% yield.[7]
Lewis acids, such as FeCl3, can also be used as the catalyst.[9] The Lewis acid catalysis process can be run at lower temperatures (50-100 °C), and gives a yield of 86%.[9]
Available forms
[edit]Diisobutyl phthalate is clear, colourless, oily liquid form with a mild odor.[10] It is insoluble in water but soluble in many organic solvents.[11]
DIBP can be sold as a pure substance or as a component of mixtures with other phthalate plasticizers or chemicals. Examples are dioctyl phthalate (DOP), diisononyl-phthalate (DINP), or bis(2-ethylhexyl) phthalate (DEHP).[12] It may be used as a component in formulations of several products including adhesives, paints, coatings and lubricants.[3] DIBP also may be present in consumer products such as toys, vinyl flooring, food packaging, and as a plasticizer or as a component of plastic formulations.[3] In many of these products DIBP is now prohibited to be used in formulations according to REACH.[12]
Environmental reactions
[edit]DIBP can undergo various reactions that may impact the environment. Examples include:
- Hydrolysis: Hydrolyzation of DIBP can be done by enzymes, bacteria, and other microorganisms in the environment to form phthalic acid and isobutyl alcohol.[13] This can lead to the breakdown and the eventual degradation of DIBP in the soil and water supply[14]
- Photodegradation: DIBP can undergo photodegradation by exposure to the sunlight. This can lead to the formation of several degradation products, including phthalic acid, isobutyraldehyde, and other aldehydes.[15]
- Biodegradation: DIBP can be degraded by microorganisms in soil and in the water. This can transform it into other compounds such as phthalic acid and various isobutyl alcohol derivatives.[16]
- Sorption: DIBP can adsorb or sorb onto soil and sediment particles, which can limit its mobility and availability for biological or chemical degradations and reactions.[17]
- Oxidation: DIBP can be oxidized in the presence of ozone or other reactive oxygen species. The formation of various oxidation products, including aldehydes, ketones, and carboxylic acids can be expected[18]
These reactions can impact the persistence, bioaccumulation, and toxicity in the environment and may have implications for human and ecosystem health.
Mechanisms of actions
[edit]PPARγ Pathway
[edit]The effects of DiBP exposure are mainly realized through its activation of peroxisome proliferator-activated receptor gamma (PPARγ).[19] PPARs are ligand-activated nuclear transcription factors, the family consists of PPARα, PPARβ/δ and PPARγ.[20] There are two isoforms of PPARγ, PPARγ2 is mainly present on cells in adipose tissue, whereas PPARγ1 is found on multiple cells like those in the gut, brain, blood vessels, and some immune and inflammatory cells. Transcriptional regulation through PPARs requires the formation of a heterodimer with retinoid X receptor (RXR). Upon activation by DiBP this PPARγ/RXR heterodimer binds to a DNA sequence called the PPAR response element (PPRE).[21] Binding of the transcription factor to this response element can result in either up- or down-regulation of genes. PPARγ is involved in lipid metabolism and storage as well as glucose metabolism through improving insulin sensitivity,[22][23] so binding of DiBP leads to altered leptin and insulin levels. DiBP also leads to a down-regulation of proteins involved in steroid production, resulting in higher levels of androgenic hormones.[19]
Cytokine-cytokine receptor pathway
[edit]Another type of pathway affected by DiBP exposure is the cytokine-cytokine receptor pathway. There are two pathways affected: the tumour necrosis factor receptor superfamily (TNFRSF) and the prolactin receptor pathway, both of which affect spermatogenesis.[19] In zebra fish, there are two types of TNFRSF: tnfrsf1a and tnfrsf1b, the latter of which is down-regulated by DiBP. Tnfrsf1b is involved in regeneration and tissue repair and its down-regulation has been shown to increase apoptosis of sperm cells.[24] Prolactin (PLR) on the other hand is up-regulated as a result of DiBP exposure.[19] Prolactin has many roles, including roles in cell regeneration and regulation of the male reproductive system.[25] High PLR concentrations as a result of phthalate exposure has been linked to reduced sperm concentrations in both adult men and zebra fish.[26][19]
Metabolism
[edit]
Upon entering circulation DiBP is quickly metabolized and excreted through urine, with metabolites reaching peak concentrations 2–4 hours after administration.[27] The main metabolite of DiBP is mono-isobutyl phthalate (MiBP), which makes up 70% of the excretion products. MiBP can be oxidized to either 2OH-mono-isobutyl phthalate (2OH-MiBP) or 3OH-mono-isobutyl phthalate (3OH-MiBP), which make up 20% and 1% of the excretion products respectively. These reactions are likely catalyzed by cytochrome P450 in the liver.[28] The ratio between MiBP and the oxidized metabolites changes depending on the amount of time that has passed since exposure.[27] The ratio between MiBP and 2OH-MiBP and that between MiBP and 3OH-MiBP show a similar trend. With the ratios being high, around 20-30:1, shortly after exposure and dropping gradually as more time passes to rest around 2-5:1. Therefore, a high ratio of oxidized metabolites to the monoester metabolite suggests that there was recent exposure to DiBP, within a few hours of measuring, while a lower ratio suggests that there has been more time since exposure. In addition to oxidation, MiBP can also undergo a glucuronidation reaction, resulting in the metabolite MiBP-glucuronide.[29]
Toxicity
[edit]There's insufficient data to determine if DIBP is associated with acute dermal or inhalation toxicity, eye or dermal irritation, or sensitization. There is evidence on DIBP being a subchronic toxicant. Exposure to the compound can induce changes in body weight, liver weight, reproductive effects, and developmental effects like testicular weight, spermatogenesis, fetal body weight, anogenital distance in male and female rats, and testicular testosterone production, among others.[30]
Biomonitoring studies show that exposures to DIBP have grown recently, presumably as a result of DIBPs use as a substitute for other phthalates such as dibutyl phthalate (DBP) in plastics.[31][32] In the United States, for instance, the prevalence of MIBP detection in urine has risen from 72% of the general population in 2001–2002 to 96% in 2009–2010, according to data from the National Health and Nutrition Examination Survey (NHANES).[32]
The main issue with phthalate exposure is typically male reproductive toxicity, which is a risk that many phthalates share.[33]
Effect on animals
[edit]A study conducted on rats shows that high dosage of DIBP administered by gavage to pregnant female rats between gestational days (GD) 6 and 20, exhibited signs of embryotoxicity and teratogenicity.[34] The growing male reproductive system was negatively impacted by DIBP, which is typical for phthalate esters. When phthalates are exposed in utero during the process of male sexual differentiation, a phenotype known as "phthalate syndrome" is created. This syndrome is characterized by underdevelopment of the male reproductive system, decreased anogenital distance (AGD), retention of the nipple in a female-like manner, and germ cell toxicity, among other things.[35][36][37] Therefore, these effects can be connected to decreased insulin-like-3 (INSL3) hormone, which controls transabdominal testicular descent, decreased androgen production in the testicles, which is essential for male sexual development, and disruption of seminiferous cord formation, Sertoli cells, and germ cell development via an unknown mode of action (MOA).[37][38][39]
Despite the limited studies in other species, research on zebrafish[40] shows that environmental exposure to DBP and DIBP can have serious consequences for fish offspring. As they go up the food chain and into polluted water, these phthalates can build up in aquatic organisms. Fish are susceptible to environmental toxins in their early lives, whether they are exposed to them directly or indirectly through their parents.
| Species | Exposure Route | Dose | Effect | Reference |
| Sprague-Dawley Rats | Oral (by gavage) | 250, 500, 750, and 1000 mg/kg/day | Pregnant females: Transient decrease in body weight gain, observed at 500 mg/kg and higher. Embryolethality and teratogenicity at 750 and 1000 mg/kg. Male fetuses: Undescended testis at 500 mg/kg. The degree of transabdominal migration of testis in relation to the bladder were disturbed at 500 mg/kg and above. | [34] |
| Zebrafish | Chemical exposure | 10, 103 and 1038 μg L−1, (LC50) was 1037.7 μg L−1 | Parental individual DIBP exposure disturbed key genes in circadian rhythm and phototransduction signal pathways, which could influence the eye development of F1 larvae. | [40] |
| Wistar Rats | Oral (by gavage) | 600 mg/kg | Disrupt fetal testicular development. AGD was reduced at GD 19 and GD 20/21 in males and increased in females exposed to DIBP. It also reduced bodyweights of male and female fetuses. | [41] |
| Sprague-Dawley Rats | Oral (by gavage) | 100, 300, 600, and 900 mg/kg/day | Reduced maternal body weight gain from 73 g in controls to 48 and 43 g in the 600 and 900 mg/kg/day dose groups, respectively. | [42] |
| Wistar Rats | Oral (by gavage) | 600 mg/kg bw/day | Reductions in fetal plasma leptin levels and in fetal insulin levels. Prenatal exposure disrupts fetal testosterone production in male rats by reducing the expression of several genes and proteins involved in steroidogenesis. In females, increases ovarian aromatase gene expression. DIBP also affected PPAR expression in the liver and testes. | [43] |
| Sprague-Dawley Rats | Oral | 100, 300, 600, or 900 mg/kg/day | Significantly reduced T production. Reduced fetal AGD, T levels, and Insl3. | [44] |
| Sprague-Dawley Rats | Oral (by gavage) | 125, 250, 500, 625 mg/kg/day | Doses ≥ 250 mg DIBP/(kg day) resulted in reduced AGD, and retained thoracic areolas/nipples at both early postnatal life, and adult necropsy. | [45] |
See also
[edit]References
[edit]- ^ Schettler, Ted (2006-02-07). "Human exposure to phthalates via consumer products". International Journal of Andrology. 29 (1): 134–139. doi:10.1111/j.1365-2605.2005.00567.x. ISSN 0105-6263. PMID 16466533.
- ^ a b c d "Diisobutyl phthalate". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. p. 10. 6782. Retrieved 2023-03-17.
- ^ a b c d "Risk Evaluation for Di-isobutyl Phthalate - (1,2-Benzene- dicarboxylic acid, 1,2- bis-(2methylpropyl) ester)". Office of Chemical Safety and Pollution Prevention (OCSPP). U.S. Environmental Protection Agency. 2020-04-14. Retrieved 2023-03-17.
- ^ "Diisobutyl phthalate". ChEMBL. ELIXIR. CHEBI:79053.
- ^ Yan Y, Zhu F, Zhu C, Chen Z, Liu S, Wang C, Gu C (October 2021). "Dibutyl phthalate release from polyvinyl chloride microplastics: Influence of plastic properties and environmental factors". Water Research. 204: 117597. Bibcode:2021WatRe.20417597Y. doi:10.1016/j.watres.2021.117597. PMID 34482095.
- ^ Hosangadi BD, Dave RH (1996-08-26). "An efficient general method for esterification of aromatic carboxylic acids". Tetrahedron Letters. 37 (35): 6375–6378. doi:10.1016/0040-4039(96)01351-2. ISSN 0040-4039.
- ^ a b Garg B, Bisht T, Ling YC (2014-10-31). "Sulfonated graphene as highly efficient and reusable acid carbocatalyst for the synthesis of ester plasticizers". RSC Advances. 4 (100): 57297–57307. Bibcode:2014RSCAd...457297G. doi:10.1039/C4RA11205A. ISSN 2046-2069.
- ^ Layek RK, Samanta S, Nandi AK (2012-03-01). "The physical properties of sulfonated graphene/poly(vinyl alcohol) composites". Carbon. 50 (3): 815–827. Bibcode:2012Carbo..50..815L. doi:10.1016/j.carbon.2011.09.039. ISSN 0008-6223.
- ^ a b Bajracharya GB, Koju R, Ojha S, Nayak S, Subedi S, Sasai H (January 2021). "Plasticizers: Synthesis of phthalate esters via FeCl3-catalyzed nucleophilic addition of alcohols to phthalic anhydride". Results in Chemistry. 3: 100190. doi:10.1016/j.rechem.2021.100190. ISSN 2211-7156. S2CID 240582041.
- ^ "Metabocard for Diisobutyl phthalate". The Human Metabolome Database (HMDB). HMDB0013835. Retrieved 2023-03-17.
- ^ "DI-ISOBUTYL PHTHALATE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2023-03-17.
- ^ a b "Phthalates - ECHA". echa.europa.eu. Retrieved 2023-03-17.
- ^ Chatterjee S, Dutta TK (September 2003). "Metabolism of butyl benzyl phthalate by Gordonia sp. strain MTCC 4818". Biochemical and Biophysical Research Communications. 309 (1): 36–43. doi:10.1016/S0006-291X(03)01513-4. PMID 12943660.
- ^ "Diisobutyl_phthalate". Hazardous Substances Data Bank (HSDB). PubChem, U.S. National Library of Medicine. 5247. Retrieved 2023-03-18.
- ^ Wang C, Zeng T, Gu C, Zhu S, Zhang Q, Luo X (2019). "Photodegradation Pathways of Typical Phthalic Acid Esters Under UV, UV/TiO2, and UV-Vis/Bi2WO6 Systems". Frontiers in Chemistry. 7: 852. doi:10.3389/fchem.2019.00852. PMC 6923729. PMID 31921775.
- ^ Lu Y, Tang F, Wang Y, Zhao J, Zeng X, Luo Q, Wang L (September 2009). "Biodegradation of dimethyl phthalate, diethyl phthalate and di-n-butyl phthalate by Rhodococcus sp. L4 isolated from activated sludge". Journal of Hazardous Materials. 168 (2–3): 938–943. Bibcode:2009JHzM..168..938L. doi:10.1016/j.jhazmat.2009.02.126. PMID 19342169.
- ^ Lu T, Xue C, Shao J, Gu JD, Zeng Q, Luo S (October 2016). "Adsorption of dibutyl phthalate on Burkholderia cepacia, minerals, and their mixtures: Behaviors and mechanisms". International Biodeterioration & Biodegradation. 114: 1–7. Bibcode:2016IBiBi.114....1L. doi:10.1016/j.ibiod.2016.05.015. ISSN 0964-8305.
- ^ Huo Y, An Z, Li M, Sun J, Jiang J, Zhou Y, He M (February 2022). "The reaction laws and toxicity effects of phthalate acid esters (PAEs) ozonation degradation on the troposphere". Environmental Pollution. 295: 118692. Bibcode:2022EPoll.29518692H. doi:10.1016/j.envpol.2021.118692. PMID 34921942. S2CID 245248168.
- ^ a b c d e Chen H, Chen K, Qiu X, Xu H, Mao G, Zhao T, et al. (November 2020). "The reproductive toxicity and potential mechanisms of combined exposure to dibutyl phthalate and diisobutyl phthalate in male zebrafish (Danio rerio)". Chemosphere. 258: 127238. Bibcode:2020Chmsp.25827238C. doi:10.1016/j.chemosphere.2020.127238. PMID 32563064. S2CID 219959568.
- ^ Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, et al. (December 2006). "International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors". Pharmacological Reviews. 58 (4): 726–741. doi:10.1124/pr.58.4.5. PMID 17132851. S2CID 2240461.
- ^ Feige JN, Gelman L, Tudor C, Engelborghs Y, Wahli W, Desvergne B (May 2005). "Fluorescence imaging reveals the nuclear behavior of peroxisome proliferator-activated receptor/retinoid X receptor heterodimers in the absence and presence of ligand". The Journal of Biological Chemistry. 280 (18): 17880–17890. doi:10.1074/jbc.M500786200. PMID 15731109.
- ^ Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J (November 2003). "Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice". Proceedings of the National Academy of Sciences of the United States of America. 100 (24): 14457–14462. Bibcode:2003PNAS..10014457K. doi:10.1073/pnas.2336090100. PMC 283613. PMID 14603033.
- ^ Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, et al. (April 2003). "Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma". Diabetes. 52 (4): 910–917. doi:10.2337/diabetes.52.4.910. PMID 12663460.
- ^ Wang YQ, Li YW, Chen QL, Liu ZH (January 2019). "Long-term exposure of xenoestrogens with environmental relevant concentrations disrupted spermatogenesis of zebrafish through altering sex hormone balance, stimulating germ cell proliferation, meiosis and enhancing apoptosis". Environmental Pollution. 244: 486–494. Bibcode:2019EPoll.244..486W. doi:10.1016/j.envpol.2018.10.079. PMID 30366296. S2CID 53112695.
- ^ Hair WM, Gubbay O, Jabbour HN, Lincoln GA (July 2002). "Prolactin receptor expression in human testis and accessory tissues: localization and function". Molecular Human Reproduction. 8 (7): 606–11. doi:10.1093/molehr/8.7.606. PMID 12087074.
- ^ Li S, Dai J, Zhang L, Zhang J, Zhang Z, Chen B (February 2011). "An association of elevated serum prolactin with phthalate exposure in adult men". Biomedical and Environmental Sciences. 24 (1): 31–39. Bibcode:2011BioES..24...31L. doi:10.3967/0895-3988.2011.01.004. PMID 21440837.
- ^ a b Koch HM, Christensen KL, Harth V, Lorber M, Brüning T (December 2012). "Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses". Archives of Toxicology. 86 (12): 1829–1839. doi:10.1007/s00204-012-0908-1. PMID 22820759. S2CID 253718517.
- ^ Carstens L, Cowan AR, Seiwert B, Schlosser D (2020). "Biotransformation of Phthalate Plasticizers and Bisphenol A by Marine-Derived, Freshwater, and Terrestrial Fungi". Frontiers in Microbiology. 11: 317. doi:10.3389/fmicb.2020.00317. PMC 7059612. PMID 32180766.
- ^ Jeong SH, Jang JH, Cho HY, Lee YB (November 2020). "Toxicokinetics of diisobutyl phthalate and its major metabolite, monoisobutyl phthalate, in rats: UPLC-ESI-MS/MS method development for the simultaneous determination of diisobutyl phthalate and its major metabolite, monoisobutyl phthalate, in rat plasma, urine, feces, and 11 various tissues collected from a toxicokinetic study". Food and Chemical Toxicology. 145: 111747. doi:10.1016/j.fct.2020.111747. PMID 32926938.
- ^ "Toxicity review of diisobutyl phthalate (DiBP)" (PDF). www.cpsc.gov. 2010. Archived from the original (PDF) on 20 April 2013. Retrieved 2023-03-17.
- ^ Wittassek M, Wiesmüller GA, Koch HM, Eckard R, Dobler L, Müller J, et al. (May 2007). "Internal phthalate exposure over the last two decades--a retrospective human biomonitoring study". International Journal of Hygiene and Environmental Health. 210 (3–4): 319–333. Bibcode:2007IJHEH.210..319W. doi:10.1016/j.ijheh.2007.01.037. PMID 17400024.
- ^ a b Zota AR, Calafat AM, Woodruff TJ (March 2014). "Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010". Environmental Health Perspectives. 122 (3): 235–241. doi:10.1289/ehp.1306681. PMC 3948032. PMID 24425099.
- ^ Yost EE, Euling SY, Weaver JA, Beverly BE, Keshava N, Mudipalli A, et al. (April 2019). "Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies". Environment International. 125: 579–594. Bibcode:2019EnInt.125..579Y. doi:10.1016/j.envint.2018.09.038. PMC 8596331. PMID 30591249.
- ^ a b Saillenfait AM, Sabaté JP, Gallissot F (August 2006). "Developmental toxic effects of diisobutyl phthalate, the methyl-branched analogue of di-n-butyl phthalate, administered by gavage to rats". Toxicology Letters. 165 (1): 39–46. doi:10.1016/j.toxlet.2006.01.013. PMID 16516415.
- ^ Foster PM, Gray Jr LE (2008). "Casarett and Doull's toxicology: The basic science of poisons". Toxicology: 761–806.
- ^ Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA, Kortenkamp A (2015). "Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission". Journal of Exposure Science & Environmental Epidemiology. 25 (4): 343–353. doi:10.1038/jes.2015.33. PMID 25944701. S2CID 19276318.
- ^ a b National Research Council (U.S.). Committee on the Health Risks of Phthalates. National Academies Press (2008). Phthalates and cumulative risk assessment : the task ahead. National Academies Press. ISBN 978-0-309-12841-4. OCLC 586808833.
- ^ Johnson KJ, Heger NE, Boekelheide K (October 2012). "Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent". Toxicological Sciences. 129 (2): 235–248. doi:10.1093/toxsci/kfs206. PMC 3491958. PMID 22700540.
- ^ Martino-Andrade AJ, Chahoud I (January 2010). "Reproductive toxicity of phthalate esters". Molecular Nutrition & Food Research. 54 (1): 148–157. doi:10.1002/mnfr.200800312. PMID 19760678.
- ^ a b Chen H, Feng W, Chen K, Qiu X, Xu H, Mao G, et al. (June 2021). "Transcriptomic responses predict the toxic effect of parental co-exposure to dibutyl phthalate and diisobutyl phthalate on the early development of zebrafish offspring". Aquatic Toxicology. 235: 105838. Bibcode:2021AqTox.23505838C. doi:10.1016/j.aquatox.2021.105838. PMID 33910148. S2CID 233447494.
- ^ Borch J, Axelstad M, Vinggaard AM, Dalgaard M (June 2006). "Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis". Toxicology Letters. 163 (3): 183–190. doi:10.1016/j.toxlet.2005.10.020. PMID 16458459.
- ^ Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. (September 2008). "A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner". Toxicological Sciences. 105 (1): 153–165. doi:10.1093/toxsci/kfn077. PMID 18411233.
- ^ Boberg J, Metzdorff S, Wortziger R, Axelstad M, Brokken L, Vinggaard AM, et al. (September 2008). "Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats". Toxicology. 250 (2–3): 75–81. Bibcode:2008Toxgy.250...75B. doi:10.1016/j.tox.2008.05.020. PMID 18602967.
- ^ Hannas BR, Lambright CS, Furr J, Evans N, Foster PM, Gray EL, Wilson VS (February 2012). "Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency". Toxicological Sciences. 125 (2): 544–557. doi:10.1093/toxsci/kfr315. PMC 3262859. PMID 22112501.
- ^ Saillenfait AM, Sabaté JP, Gallissot F (October 2008). "Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat". Reproductive Toxicology. 26 (2): 107–115. Bibcode:2008RepTx..26..107S. doi:10.1016/j.reprotox.2008.07.006. PMID 18706996.


 French
French Deutsch
Deutsch