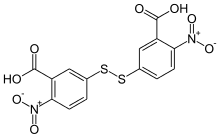
Ellman's reagent
 | |
| Names | |
|---|---|
| Preferred IUPAC name 5,5′-Disulfanediylbis(2-nitrobenzoic acid) | |
| Other names 3,3′-Disulfanediylbis(6-nitrobenzoic acid) 5-(3-Carboxy-4-nitrophenyl)disulfanyl-2-nitrobenzoic acid Dithionitrobenzoic acid 5,5′-Dithiobis(2-nitrobenzoic acid) | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | DTNB |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.650 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C14H8N2O8S2 | |
| Molar mass | 396.34 g·mol−1 |
| Melting point | 240 to 245 °C (464 to 473 °F; 513 to 518 K) (decomposes) |
| Hazards | |
| GHS labelling:[2] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB) is a colorogenic chemical used to quantify the number or concentration of thiol groups in a sample.[3] It was developed by George L. Ellman.
Preparation
[edit]In Ellman's original paper,[3] he prepared this reagent by oxidizing 2-nitro-5-chlorobenzaldehyde to the carboxylic acid, introducing the thiol via sodium sulfide, and coupling the monomer by oxidization with iodine. Today, this reagent is readily available commercially.
Ellman's test
[edit]Thiols react with this compound, cleaving the disulfide bond to give 2-nitro-5-thiobenzoate (TNB−), which ionizes to the TNB2− dianion in water at neutral and alkaline pH. This TNB2− ion has a yellow color.
This reaction is rapid and stoichiometric, with the addition of one mole of thiol releasing one mole of TNB. The TNB2− is quantified in a spectrophotometer by measuring the absorbance of visible light at 412 nm, using an extinction coefficient of 14,150 M−1 cm−1 for dilute buffer solutions,[4][5] and a coefficient of 13,700 M−1 cm−1 for high salt concentrations, such as 6 M guanidinium hydrochloride or 8 M urea.[5] Ellman's original 1959 publication estimated the molar extinction at 13,600 M−1 cm−1, and this value can be found in some modern applications of the method despite improved determinations.[6] Commercial DTNB may not be completely pure, so may require recrystallization to obtain completely accurate and reproducible results.[5]
Ellman's reagent can be used for measuring low-molecular mass thiols such as glutathione in both pure solutions and biological samples, such as blood.[7] It can also measure the number of thiol groups on proteins.[7]
References
[edit]- ^ 5,5′-Dithiobis(2-nitrobenzoic acid) at Sigma-Aldrich
- ^ "5,5'-Dithiobis(2-nitrobenzoic acid)". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.
- ^ a b Ellman GL (1959). "Tissue sulfhydryl groups". Arch. Biochem. Biophys. 82 (1): 70–7. doi:10.1016/0003-9861(59)90090-6. PMID 13650640.
- ^ Collier HB (1973). "Letter: A note on the molar absorptivity of reduced Ellman's reagent, 3-carboxylato-4-nitrothiophenolate". Anal. Biochem. 56 (1): 310–1. doi:10.1016/0003-2697(73)90196-6. PMID 4764694.
- ^ a b c Riddles PW, Blakeley RL, Zerner B (1983). "Reassessment of Ellman's reagent". Enzyme Structure Part I. Methods in Enzymology. Vol. 91. pp. 49–60. doi:10.1016/S0076-6879(83)91010-8. ISBN 978-0-12-181991-0. PMID 6855597.
- ^ Riener CK, Kada G, Gruber HJ (2002). "Quick measurement of protein sulfhydryls with Ellman's reagent and with 4,4'-dithiodipyridine". Anal Bioanal Chem. 373 (4–5): 266–76. doi:10.1007/s00216-002-1347-2. PMID 12110978. S2CID 30366479.
- ^ a b Riener, Christian K.; Kada, Gerald; Gruber, Hermann J. (2002-07-01). "Quick measurement of protein sulfhydryls with Ellman's reagent and with 4,4′-dithiodipyridine". Analytical and Bioanalytical Chemistry. 373 (4–5): 266–276. doi:10.1007/s00216-002-1347-2. ISSN 1618-2642. PMID 12110978. S2CID 30366479.
External links
[edit]- Quantitation of sulfhydryls DTNB, Ellman’s reagent (uses incorrect absorbance coefficient)


 French
French Deutsch
Deutsch