Quingestanol
Chemical compound
This article is about a non-clinically used progestin compound. For the pharmaceutical drug, see quingestanol acetate.
 | |
| Clinical data | |
|---|---|
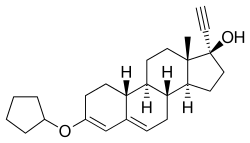
| Other names | Norethisterone 3-cyclopentyl enol ether; 3-(Cyclopentyloxy)-17α-ethynylestra-3,5-dien-17β-ol |
| Drug class | Progestin; Progestogen |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.031.078 |
| Chemical and physical data | |
| Formula | C25H34O2 |
| Molar mass | 366.545 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Quingestanol (INNTooltip International Nonproprietary Name, BANTooltip British Approved Name), also known as norethisterone 3-cyclopentyl enol ether, is a progestin of the 19-nortestosterone group which was never marketed.[1] It is a prodrug of norethisterone.[2][3] An acylated derivative, quingestanol acetate, is used as a pharmaceutical drug.[1]
See also
[edit]References
[edit]- ^ a b Macdonald F (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1721. ISBN 978-0-412-46630-4. Retrieved 12 May 2012.
- ^ Raynaud JP, Ojasoo T (1986). "The design and use of sex-steroid antagonists". J. Steroid Biochem. 25 (5B): 811–33. doi:10.1016/0022-4731(86)90313-4. PMID 3543501.
Similar androgenic potential is inherent to norethisterone and its prodrugs (norethisterone acetate, ethynodiol diacetate, lynestrenol, norethynodrel, quingestanol).
- ^ Di Carlo FJ, Loo JC, Aceto T, Zuleski FR, Barr WH (1974). "Quingestanol acetate metabolism in women". Pharmacology. 11 (5): 287–303. doi:10.1159/000136501. PMID 4853997.
| ARTooltip Androgen receptor |
| ||||||
|---|---|---|---|---|---|---|---|
| GPRC6A |
| ||||||
This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it. |


 French
French Deutsch
Deutsch