Thelytoky

Thelytoky (from the Greek θῆλυς thēlys "female" and τόκος tókos "birth") is a type of parthenogenesis and is the absence of mating and subsequent production of all female diploid offspring as for example in aphids. Thelytokous parthenogenesis is rare among animals and reported in about 1,500 species, about 1 in 1000 of described animal species, according to a 1984 study.[1] It is more common in invertebrates, like arthropods, but it can occur in vertebrates, including salamanders, fish, and reptiles such as some whiptail lizards.
Thelytoky can occur by different mechanisms, each of which has a different impact on the level of homozygosity. It is found in several groups of Hymenoptera, including Apidae, Aphelinidae, Cynipidae, Formicidae, Ichneumonidae, and Tenthredinidae.[2] It can be induced in Hymenoptera by the bacteria Wolbachia and Cardinium.[3]
Advantages of thelytoky
[edit]Species may encounter a few advantages employing this form of mating system. Thelytoky allows females to pass along genotypes that ensure success in that particular environment, having only daughters increases the species output, and energy that would otherwise be exerted into finding or attracting a mate can directly be invested in reproduction.[4]
Thelytoky can occur naturally, or it can be induced by scientists in a laboratory setting.[5] In some species, thelytoky can also occur through the fusion of two female gametes.[6]
Types of thelytoky
[edit]Facultative thelytoky refers to an individual being capable of reproducing sexually or asexually depending on environmental conditions. For example, smalltooth sawfish in Florida populations can be facultatively thelytokous, meaning that they will reproduce sexually when conditions are favorable, but switch to thelytoky when resources and mates become scarce.[7]
Accidental thelytoky occurs when a female organism produces offspring asexually due to the absence or failure of fertilization by a male. This can occur in species that normally reproduce sexually but are unable to find a mate, or in species in which mating is unsuccessful due to physical or behavioral barriers. While accidental thelytoky can provide a short-term reproductive solution in the absence of a mate, it is typically not sustainable over the long-term due to the loss of genetic diversity.[8]
Cyclical thelytoky is a form of thelytoky in which organisms alternate between sexual and asexual reproduction in a regular cycle. This type of reproduction is seen in cynipid gall wasps, in which sexual reproduction occurs in alternate generations. The asexual reproduction that occurs in between these sexual generations is typically facilitated by the presence of specific environmental cues, such as temperature or photoperiod. The genetic diversity generated by sexual reproduction in these organisms is thought to play an important role in their ability to adapt to changing environmental conditions.[9]
Obligate thelytoky refers to a form of asexual reproduction in which an individual is unable to reproduce sexually and must rely on asexual reproduction for reproduction. Species that are obligately thelytokous do not have the genetic or physiological mechanisms necessary to produce males, and thus rely solely on female offspring to perpetuate their lineage. Examples of obligately thelytokous species include some members of cerapachys ants and some species of whiptail lizards.[10]
Arrhenotoky and thelytoky in Hymenoptera
[edit]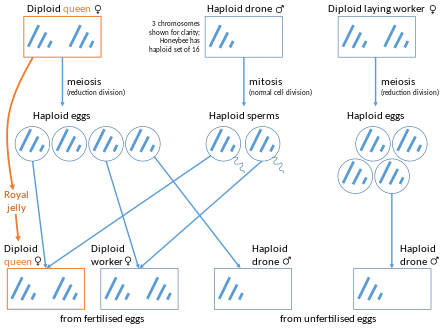
Hymenoptera (ants, bees, wasps, and sawflies) have a haplodiploid sex-determination system. They produce haploid males from unfertilized eggs (arrhenotoky), a form of parthenogenesis. However, in a few social hymenopterans, queens or workers are capable of producing diploid female offspring by thelytoky.[11] The daughters produced may or may not be complete clones of their mother depending on the type of parthenogenesis that takes place.[12][13] The offspring can develop into either queens or workers. Examples of such species include the Cape bee, Apis mellifera capensis, Mycocepurus smithii and clonal raider ant, Ooceraea biroi.
Automixis
[edit]
Automixis is a form of thelytoky. In automictic parthenogenesis, meiosis takes place and diploidy is restored by fusion of first division non-sister nuclei (central fusion) or the second division sister nuclei (terminal fusion).[14] (see diagram).
With central fusion
[edit]Automixis with central fusion tends to maintain heterozygosity in the passage of the genome from mother to daughter. This form of automixis has been observed in several ant species including the desert ant Cataglyphis cursor,[11] the clonal raider ant Cerapachys biroi,[15] the predaceous ant Platythyrea punctata,[14] and the electric ant (little fire ant) Wasmannia auropunctata.[16] Automixis with central fusion also occurs in the Cape honey bee Apis mellifera capensis,[13] the brine shrimp Artemia parthenogenetica,[17] and the termite Embiratermes neotenicus.[18]
Oocytes that undergo automixis with central fusion often display a reduced rate of crossover recombination. A low rate of recombination in automictic oocytes favors maintenance of heterozygosity, and only a slow transition from heterozygosity to homozygosity over successive generations. This allows avoidance of immediate inbreeding depression. Species that display central fusion with reduced recombination include the ants P. punctata[14] and W. auropunctata,[16] the brine shrimp A. parthenogenetica,[17] and the honey bee A. m. capensis.[13] In A. m. capensis, the recombination rate during the meiosis associated with thelytokous parthenogenesis is reduced by more than 10-fold.[13] In W. auropunctata the reduction is 45-fold.[16]
Single queen colonies of the narrow headed ant Formica exsecta provide an illustrative example of the possible deleterious effects of increased homozygosity. In this ant the level of queen homozygosity is negatively associated with colony age.[19] Reduced colony survival appears to be due to decreased queen lifespan resulting from queen homozygosity and expression of deleterious recessive mutations (inbreeding depression).
With terminal fusion
[edit]Automixis with terminal fusion tends to promote homozygosity in the passage of the genome from mother to daughter. This form of automixis has been observed in the water flea Daphnia magna[20] and the Colombian rainbow boa constrictor Epicrates maurus.[21] Parthenogenesis in E. maurus is only the third genetically confirmed case of consecutive virgin births of viable offspring from a single female within any vertebrate lineage.[21] However, survival of offspring over two successive litters was poor, suggesting that automixis with terminal fusion leads to homozygosity and expression of deleterious recessive alleles (inbreeding depression).
See also
[edit]References
[edit]- ^ White, Michael J.D. (1984). "Chromosomal Mechanisms in Animal Reproduction". Bolletino di Zoologia. 51 (1–2): 1–23. doi:10.1080/11250008409439455. ISSN 0373-4137.
- ^ Suomalainen, Esko; Anssi Saura; Juhani Lokki (1987-08-31). Cytology and evolution in parthenogenesis. CRC Press. pp. 29–31, 51. ISBN 978-0-8493-5981-1.
- ^ Jeong, G; R Stouthamer (2004-11-03). "Genetics of female functional virginity in the Parthenogenesis-Wolbachia infected parasitoid wasp Telenomus nawai (Hymenoptera: Scelionidae)". Heredity. 94 (4): 402–407. doi:10.1038/sj.hdy.6800617. ISSN 0018-067X. PMID 15523503.
- ^ J., Bell, William (2007). Cockroaches : ecology, behavior, and natural history. Johns Hopkins University Press. ISBN 978-1-4356-9271-8. OCLC 646769575.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Zhu, Dao-Hong; He, Yi-Yuan; Fan, Yong-Sheng; Ma, Ming-Yong; Peng, De-Liang (September 2007). "Negative evidence of parthenogenesis induction by Wolbachia in a gallwasp species, Dryocosmus kuriphilus". Entomologia Experimentalis et Applicata. 124 (3): 279–284. doi:10.1111/j.1570-7458.2007.00578.x. ISSN 0013-8703. S2CID 84922476.
- ^ Benjamin P Oldroyd; Michael H Allsopp; Rosalyn S Gloag; Julianne Lim; Lyndon A Jordan; Madeleine Beekman (September 1, 2008). "Thelytokous Parthenogenesis in Unmated Queen Honeybees (Apis mellifera capensis): Central Fusion and High Recombination Rates". Genetics. 180 (1): 359–366. doi:10.1534/genetics.108.090415. PMC 2535687. PMID 18716331.
- ^ Fields, Andrew T.; Feldheim, Kevin A.; Poulakis, Gregg R.; Chapman, Demian D. (2015-06-01). "Facultative parthenogenesis in a critically endangered wild vertebrate". Current Biology. 25 (11): R446–R447. doi:10.1016/j.cub.2015.04.018. ISSN 0960-9822. PMID 26035783.
- ^ Pardo, M C; López-León, M D; Cabrero, J; Camacho, J P M (November 1995). "Cytological and developmental analysis of tychoparthenogenesis in Locusta migratoria". Heredity. 75 (5): 485–494. doi:10.1038/hdy.1995.165. ISSN 0018-067X.
- ^ "Correction to: 'Closing the life cycle' of Andricus quercuslanigera (Hymenoptera: Cynipidae)". Annals of the Entomological Society of America. 116 (1): 72–73. 2022-10-22. doi:10.1093/aesa/saac020. ISSN 0013-8746.
- ^ Cuellar, Orlando (1968). "Additional Evidence for True Parthenogenesis in Lizards of the Genus Cnemidophorus". Herpetologica. 24 (2): 146–150. ISSN 0018-0831. JSTOR 3891303.
- ^ a b Pearcy, M. (2004). "Conditional Use of Sex and Parthenogenesis for Worker and Queen Production in Ants" (PDF). Science. 306 (5702): 1780–1783. Bibcode:2004Sci...306.1780P. doi:10.1126/science.1105453. PMID 15576621. S2CID 37558595.
- ^ Fournier, Denis; Estoup, Arnaud; Orivel, Jérôme; Foucaud, Julien; Jourdan, Hervé; Breton, Julien Le; Keller, Laurent (2005). "Clonal reproduction by males and females in the little fire ant" (PDF). Nature. 435 (7046): 1230–1234. Bibcode:2005Natur.435.1230F. doi:10.1038/nature03705. PMID 15988525. S2CID 1188960.
- ^ a b c d Baudry E, Kryger P, Allsopp M, Koeniger N, Vautrin D, Mougel F, Cornuet JM, Solignac M (2004). "Whole-genome scan in thelytokous-laying workers of the Cape honeybee (Apis mellifera capensis): central fusion, reduced recombination rates and centromere mapping using half-tetrad analysis". Genetics. 167 (1): 243–252. doi:10.1534/genetics.167.1.243. PMC 1470879. PMID 15166151.
- ^ a b c Kellner, Katrin; Heinze, Jürgen (2010). "Mechanism of facultative parthenogenesis in the ant Platythyrea punctata". Evolutionary Ecology. 25 (1): 77–89. doi:10.1007/s10682-010-9382-5. S2CID 24645055.
- ^ Oxley PR, Ji L, Fetter-Pruneda I, McKenzie SK, Li C, Hu H, Zhang G, Kronauer DJ (2014). "The genome of the clonal raider ant Cerapachys biroi". Current Biology. 24 (4): 451–8. doi:10.1016/j.cub.2014.01.018. PMC 3961065. PMID 24508170.
- ^ a b c Rey O, Loiseau A, Facon B, Foucaud J, Orivel J, Cornuet JM, Robert S, Dobigny G, Delabie JH, Mariano Cdos S, Estoup A (2011). "Meiotic recombination dramatically decreased in thelytokous queens of the little fire ant and their sexually produced workers". Molecular Biology and Evolution. 28 (9): 2591–601. doi:10.1093/molbev/msr082. PMID 21459760.
- ^ a b Nougué O, Rode NO, Jabbour-Zahab R, Ségard A, Chevin LM, Haag CR, Lenormand T (2015). "Automixis in Artemia: solving a century-old controversy". Journal of Evolutionary Biology. 28 (12): 2337–48. doi:10.1111/jeb.12757. PMID 26356354.
- ^ Fougeyrollas R, Dolejšová K, Sillam-Dussès D, Roy V, Poteaux C, Hanus R, Roisin Y (2015). "Asexual queen succession in the higher termite Embiratermes neotenicus". Proceedings of the Royal Society of London B: Biological Sciences. 282 (1809): 20150260. doi:10.1098/rspb.2015.0260. PMC 4590441. PMID 26019158.
- ^ Haag-Liautard C, Vitikainen E, Keller L, Sundström L (2009). "Fitness and the level of homozygosity in a social insect" (PDF). Journal of Evolutionary Biology. 22 (1): 134–42. doi:10.1111/j.1420-9101.2008.01635.x. PMID 19127611.
- ^ Svendsen N, Reisser CM, Dukić M, Thuillier V, Ségard A, Liautard-Haag C, Fasel D, Hürlimann E, Lenormand T, Galimov Y, Haag CR (2015). "Uncovering cryptic asexuality in Daphnia magna by RAD sequencing". Genetics. 201 (3): 1143–55. doi:10.1534/genetics.115.179879. PMC 4649641. PMID 26341660.
- ^ a b Booth W, Million L, Reynolds RG, Burghardt GM, Vargo EL, Schal C, Tzika AC, Schuett GW (2011). "Consecutive virgin births in the new world boid snake, the Colombian rainbow boa, Epicrates maurus". Journal of Heredity. 102 (6): 759–63. doi:10.1093/jhered/esr080. PMID 21868391.


 French
French Deutsch
Deutsch