Unoprostone
 | |
| Clinical data | |
|---|---|
| Trade names | Rescula |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 14 min |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.227.145 |
| Chemical and physical data | |
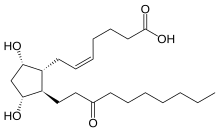
| Formula | C22H38O5 |
| Molar mass | 382.541 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Unoprostone (INN) is a prostaglandin analogue. Its isopropyl ester, unoprostone isopropyl, was marketed under the trade name Rescula for the management of open-angle glaucoma and ocular hypertension.[1][2]
It was approved by the Food and Drug Administration in 2000.[3]
In 2009, Sucampo Pharmaceuticals acquired the rights to the drug in the U.S. and Canada.[4]
In 2015, the drug was discontinued in the U.S.[citation needed]
References
[edit]- ^ Micromedex Detailed Consumer Information
- ^ Fung DS, Whitson JT (2014). "An evidence-based review of unoprostone isopropyl ophthalmic solution 0.15% for glaucoma: place in therapy". Clinical Ophthalmology. 8. Auckland, N.Z.: 543–54. doi:10.2147/OPTH.S41562. PMC 3958522. PMID 24648719.
- ^ "Drug Approval Package". Food and Drug Administration.
- ^ "Sucampo Pharmaceuticals, Inc. Acquires Rights to Rescula for U.S. and Canada" (Press release). Business Wire. April 24, 2009.


 French
French Deutsch
Deutsch