Vilsmeier reagent
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1-Chloro-N,N-dimethylmethaniminium chloride | |
| Other names (chloromethylene)dimethyliminium chloride | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.102.443 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C3H7Cl2N | |
| Molar mass | 128.00 g·mol−1 |
| Appearance | white solid |
| Melting point | 132 °C (270 °F; 405 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H290, H302, H314, H360 | |
| P201, P202, P234, P260, P264, P270, P280, P281, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P321, P330, P363, P390, P404, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
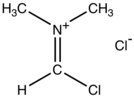
The Vilsmeier reagent is an organic compound with the formula [(CH3)2NCHCl]Cl. It is a salt consisting of the N,N-dimethyliminium cation ([(CH3)2N=CHCl]+) and chloride anion. Depending on the particular reaction, the anion can vary. In typical POCl3-based reactions, the anion is PO2Cl2−. The iminium cation [(CH3)2N=CHCl]+ is the reactive component of interest. This iminium species is a derivative of the imidoyl chloride CH3N=CHCl. Analogues of this particular reagent are generated when tertiary amides other than DMF are treated with POCl3.
The salt is a white solid that is soluble in polar organic solvents. Vilsmeier reagent is the active intermediate in the formylation reactions, the Vilsmeier reaction or Vilsmeier-Haack reaction that use mixtures of dimethylformamide and phosphorus oxychloride to generate the Vilsmeier reagent, which in turn attacks a nucleophilic substrate and eventually hydrolyzes to give formyl. It is a source of "O=CH+".[1]
See also
[edit]- Eschenmoser's salt, [(CH3)2NCH2]I
References
[edit]- ^ Paul R. Giles; Charles M. Marson (2001). "Dimethylchloromethyleneammonium Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd319m. ISBN 0-471-93623-5.
- ^ Jones, G.; Stanforth, S. P. (2000). "The Vilsmeier Reaction of Non-Aromatic Compounds". Org. React. 56 (2): 355–686. doi:10.1002/0471264180.or056.02.


 French
French Deutsch
Deutsch