Permanganate - Simple English Wikipedia, the free encyclopedia
 | |
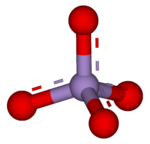 | |
| Names | |
|---|---|
| Systematic IUPAC name Permanganate | |
| Identifiers | |
| Properties | |
| MnO− 4 | |
| Molar mass | 118.934 g mol-1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Permanganate is an ion that contains one manganese ion in an oxidation state of +7 and 4 oxide atoms. It is a strong oxidizing agent. Permanganates are purple-black. Permanganates stain because they are easily reduced to brown-black manganese dioxide.
Examples
[change | change source]Related pages
[change | change source]- Chromate, a similar ion
- Manganese
- Manganese dioxide
Sources
[change | change source]


 French
French Deutsch
Deutsch