Selenous acid - Simple English Wikipedia, the free encyclopedia
 | |
 | |
| Names | |
|---|---|
| IUPAC names Selenous acid Selenic(IV) acid | |
| Other names Selenious acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.067 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| Properties | |
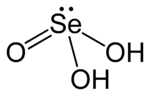
| H2SeO3 | |
| Molar mass | 128.97 g/mol |
| Appearance | white hygroscopic crystals |
| Density | 3.0 g/cm3 |
| Melting point | decomposes at 70°C |
| very soluble | |
| Solubility | soluble in ethanol |
| Acidity (pKa) | 2.46, 7.3[2] |
| Conjugate base | Hydrogenselenite |
| −45.4·10−6 cm3/mol | |
| Related compounds | |
| Other anions | {{{value}}} |
| Other cations | {{{value}}} |
| Related compounds | {{{value}}} |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Selenous acid, also known as selenious acid, is a chemical compound. Its chemical formula is H2SeO3. It is an acid. It contains hydrogen and selenite ions.
Properties
[change | change source]Selenous acid is a weak acid. It can be heated to make selenium dioxide. It is more stable than sulfurous acid. It can be crystallized as a white solid. It is a weak oxidizing agent. It reacts with bases to make selenites.
Preparation
[change | change source]It is made by dissolving selenium dioxide in water.
Uses
[change | change source]It is used to dye steel a blue-grey color. It is used in the making of organic compounds.
Safety
[change | change source]Selenous acid is very toxic. Just ingesting a small amount can kill you.
Related pages
[change | change source]Sources
[change | change source]- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–81. ISBN 0-8493-0594-2.
- ↑ Ka and pKa for Polyprotic Acids. ucdsb.on.ca


 French
French Deutsch
Deutsch